
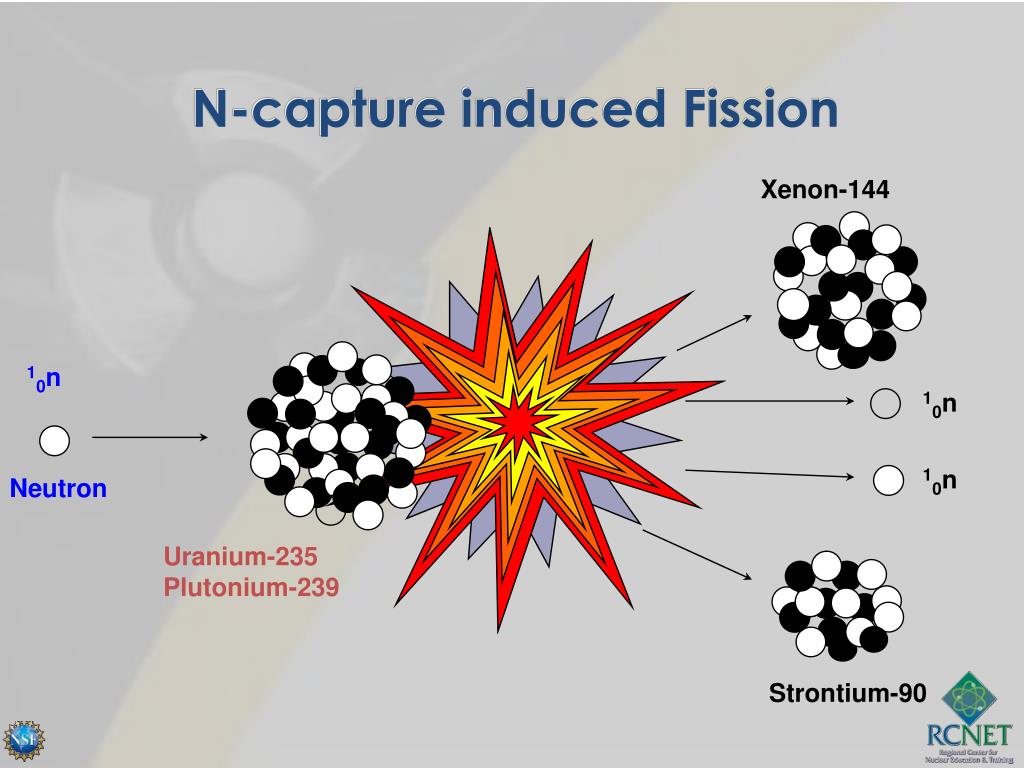

Name 6 types of simple machines that make work easier AND an example of each 1._ 2._ 3._ 4._ 5._ 6._ Round the number to the place value indicated. could you please help meĮxpressed to the correct number of significant figures, the sum of two masses is 445.2 grams. 3) (devenir) ce professeur _ _ directeur. 2) (venir) La pharmacienne _ _ parler aux eleves. 1) (naitre) Notre directrice _ _ en 1975. If the number of moles of a reactant is provided, fill in the required amount of theĬomplete les phrases avec la forme correcte du verbe au passe compose. The rate constant of the reaction is _Ĭonsider the following balanced equation: 2N2H4(g)+N2O4(g)→3N2(g)+4H2O(g) Complete the following table showing the appropriate number of moles of reactants and products. They can be absorbed by control rods.Ī first-order reaction is 35.5% complete in 4.90 minutes. They can initiate other fission reactions. They are converted from mass into energy. What happens to the neutrons produced in a fission reaction? Select all that apply. No, it is not because the mass number does not change. The model shows alpha decay, which is a type of nuclear fission. Here's the answers for the quick check I took 1. What is a commonly used isotope in a nuclear fission reactor? Describe how a fission nuclear reactor power plant works. There are two main types of process:ĭescribe what happens in a fission reaction. Because this process does not require oxygen, it is said to be _(5)_. Write the nuclear equation for this reaction.Ĭalculate the energy released when 0.5g of uranium 235 undergoes a fission reaction.Įquation of nuclear fission of urnaium 235 forming iodine 131ĭuring the process of _(1)_, cells convert _(2)_ to NAD+ by passing high-energy _(3)_ back to _(4)_ acid. In a fission reaction, uranium-235 bombarded with a neutron produces strontium-94, another small nucleus, and 3 neutrons. 1/0n +235/92U -> 137/52 Te +?/?X + 2 1/0 n + energy also how do you decipher whether its 2 1/0n rather than 3? Supply the missing atomic symbol to complete the equation for the following nuclear fission reaction. The mass of the U-235 is 235 amu and the mass of the neutron is In the first step of nuclear fission, a U-235 atom absorbs a neutron and becomes U-236 before becoming unstable and breaking into two smaller atoms and emitting various energies and particles.


 0 kommentar(er)
0 kommentar(er)
